RESEARCH
Coevolution and Mechanical Function in Collagen
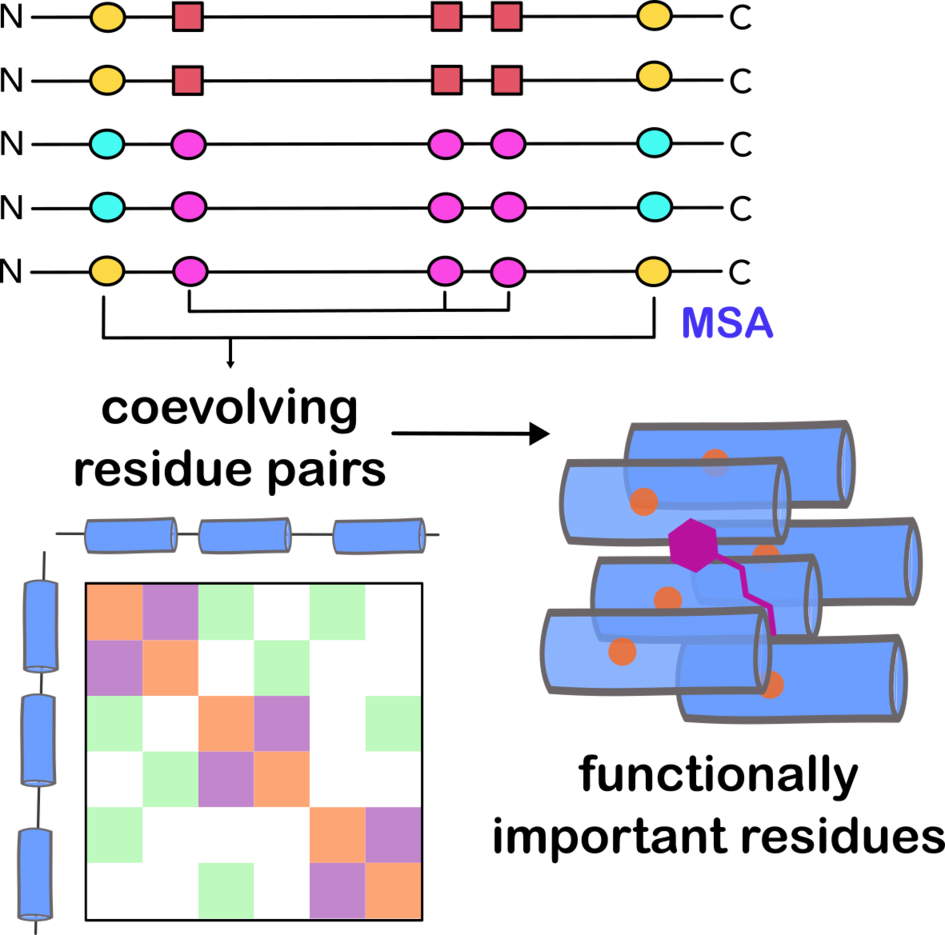
Collagen is one of the most abundant proteins in the extracellular matrix and is essential for tissue structure and function. Its unique triple-helical structure is key to the mechanical properties of different tissues, from the elasticity of skin to the strength of bones. In our research, we explore the evolutionary history of collagen, focusing on how natural selection has shaped its amino acid sequence to adapt to mechanical forces.
Using advanced bioinformatics tools like Direct Coupling Analysis, we identify coevolved residue pairs within collagen sequences. This approach allows us to examine collagen across a range of species and tissue types, uncovering patterns of evolution that have remained consistent or changed over time. We pay special attention to residues that interact between triple helices, those involved in crosslinking, and amino acids with potential redox activity. This analysis gives us a deeper understanding of how intra- and inter-chain interactions contribute to collagen’s unique mechanical properties.
Our goal is to develop a predictive model that links collagen’s evolutionary markers to its mechanical behavior. Such a model would not only enhance our understanding of collagen’s role in tissue mechanics but could also inform biomaterial design and advance our knowledge of collagen-related disorders.
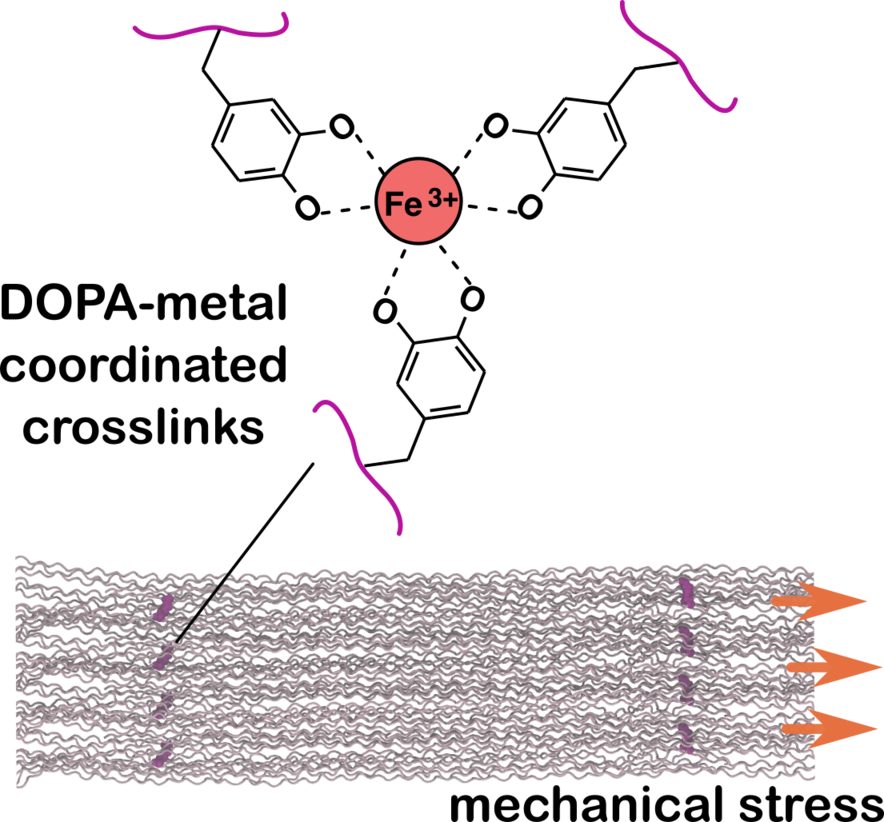
Metal Ions and DOPA in Collagen Crosslinking
Collagen's covalent crosslinks have been a focus of research for years, but there's still much to learn about this complex protein. We're exploring a less-studied aspect: the potential role of metal-coordinated crosslinks. We're especially intrigued by crosslinks involving DOPA (3,4-dihydroxy-L-phenylalanine) residues.
Our interest in this area was sparked by the fascinating properties of mussel byssus threads. These tough, elastic fibers use DOPA-Fe³⁺ coordination crosslinks to achieve their remarkable strength. We hypothesize that similar mechanisms might exist in collagen, potentially contributing to its mechanical properties.
To investigate this hypothesis, we employ a combination of computational and experimental methods. Our approach includes simulations to model the formation and behavior of these crosslinks at the molecular level and measurements to detect and characterize these metal-coordinated structures in collagen samples.
Our research aims to elucidate how these metal-coordinated crosslinks might influence collagen's mechanical properties, particularly its ability to withstand stress and potentially self-heal. Understanding these mechanisms could provide new insights into collagen's structure-function relationships and inform the development of biomaterials with enhanced mechanical properties.
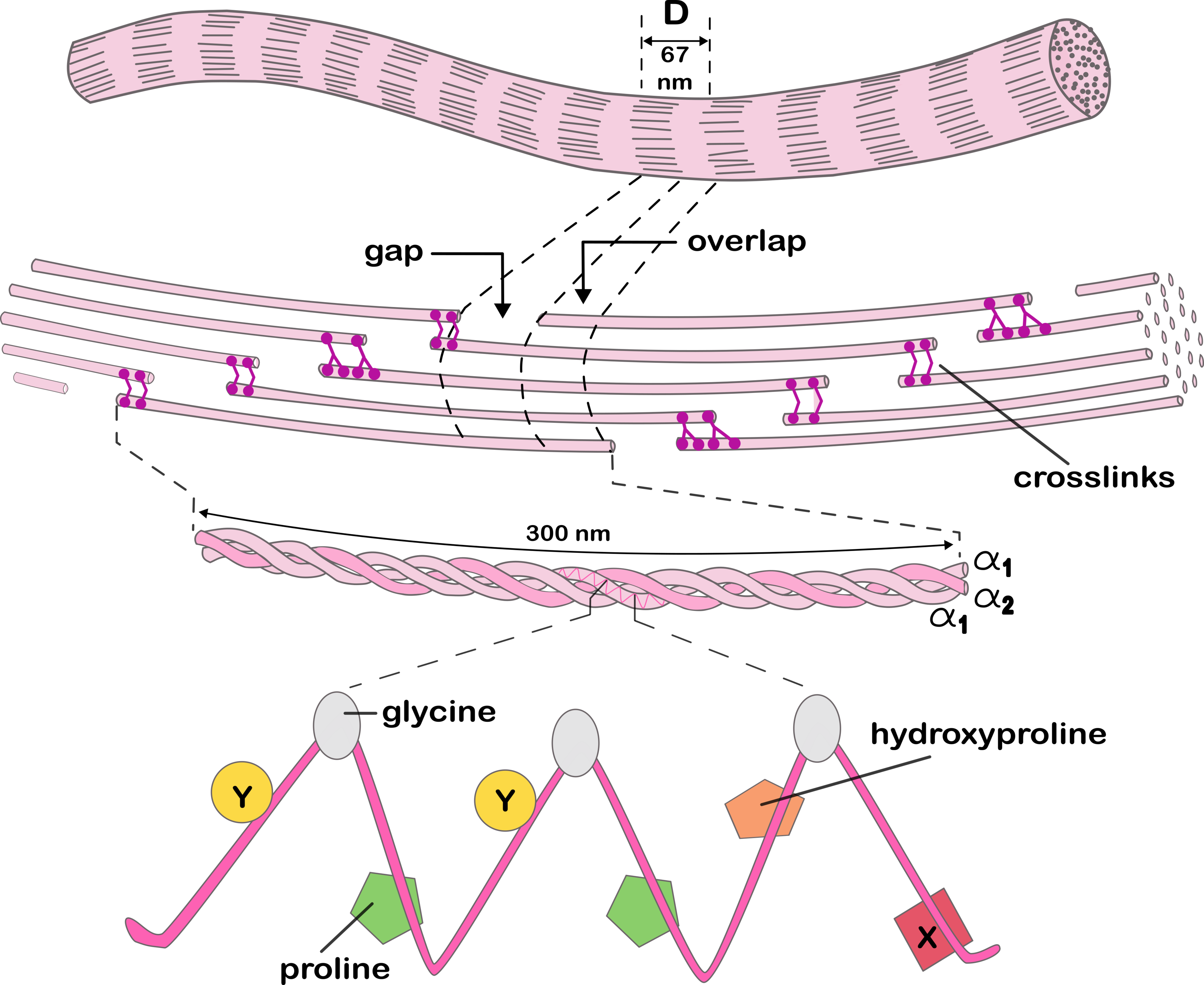
Force Propagation in Collagen
Understanding how mechanical forces propagate through collagen's complex structure is crucial for predicting its behavior under stress. Our research focuses on developing and refining full-atom models of collagen, integrating insights from our studies on collagen evolution and crosslinking mechanisms.
With these computational models, we can explore how different parts of collagen's structure affect how force spreads through it. We're looking at things like where crosslinks are located, how the triple helix is shaped, and how hydrogen bonds are arranged.
A key challenge in collagen research is bridging the gap between molecular-level interactions and macroscale mechanical properties. Our work aims to address this by developing more accurate predictive models of collagen behavior under various mechanical conditions.
FUNDING

